Abstract
Introduction: Limited real-world data exist on treatments for relapsed/refractory multiple myeloma (RRMM) since the approval of several new agents. This study assessed treatment patterns and outcomes of patients with RRMM receiving ≥2 lines of therapy in US community oncology practices.
Methods: A chart review was conducted in patients ≥18 years with MM diagnosed January 1, 2011-May 31, 2017, from a large electronic medical record database. Patient data were examined from the date of initiation of first-line therapy (1LT) for MM until death, loss to follow-up, or study end date, whichever was earliest. This study was hypothesis generating, thus no statistical tests were performed. Descriptive statistics were used to describe baseline demographic/clinical characteristics, treatment patterns, median progression-free survival (mPFS) and median overall survival (mOS) for the overall RRMM population. Patients were then stratified into "older" vs. "newer" treatment cohorts based on whether the drugs used in each line of therapy were approved before, or after 2013.
Results: Of 1005 charts reviewed, 456 patients had received ≥2 lines of therapy and were included in the chart review study (median age at diagnosis: 70.4 years; females: 39.5%, males 60.5%; bone involvement at diagnosis: 66.0%; International Staging System stage within 1 month of diagnosis: I 28.7%, II 27.9%, III 43.4%; 183 (40.1%) patients received 3LT, 75 (16.4%) 4LT, and 29 (6.4%) 5LT. 1LT was dominated by bortezomib (BTZ), lenalidomide (LEN), and the combination of the two, with 93.3% of patients using these agents as 1LT and 69.8% of patients as 2LT. In 3LT and beyond, there was greater use of newly approved drugs (approved since 2013) compared with 1LT and 2LT; pomalidomide (14.8% 3LT, 17.3% 4LT, 17.2% 5LT), carfilzomib (19.1% 3LT, 13.3% 4LT, 17.2% 5LT), elotuzumab (2.2% 3LT, 4.0% 4LT, 3.4% 5LT), panobinostat (0% 3LT, 2.7% 4LT, 3.4% 5LT), daratumumab (6.6% 3LT, 13.3% 4LT, 17.2% 5LT), and ixazomib (0% 3LT, 1.3% 4LT, 3.4% 5LT) with the exception of the BTZ/LEN combination. However, patients receiving either BTZ, LEN, or both in combination as 1LT or 2LT often received the agent(s) as re-treatment in lines 2-6 (46.2%-55.6%). Median time on treatment decreased from 7.5 months in 1LT to ≤2.3 months in 4LT and 5LT, and median treatment-free intervals decreased from 1.6 months between 1LT and 2LT to 0.5 months between 4LT and 5LT. The most common reason for discontinuation was disease progression and drug toxicity/intolerability.
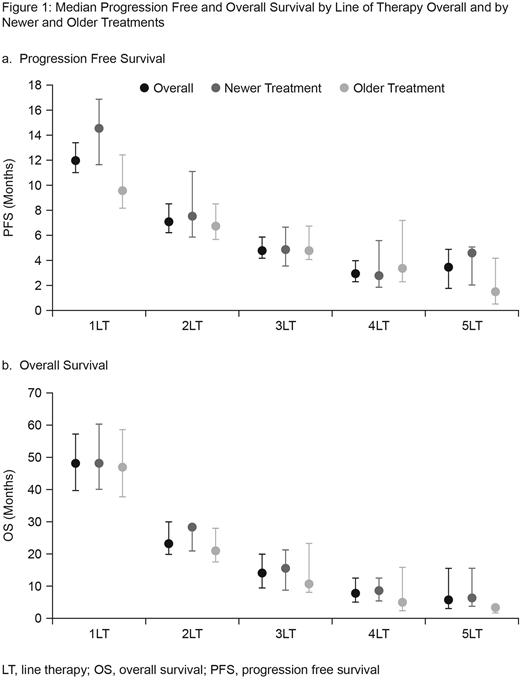
The most commonly reported adverse events (AEs) for all lines of therapy were fatigue (71.6%-78.3%), bone pain (38.5%-69.1%) and anemia (53.8%-69.3%). AEs were generally constant across lines of therapy, except for bone pain, anemia, and neuropathy, which broadly decreased with increasing line of therapy. Overall, mPFS ranged from 12.0 months in 1LT to 3.5 months in 5LT and mOS ranged from 48.2 months in 1LT to 5.8 months in 5LT (Figure 1). A trend for increased mPFS and mOS with newer vs. older drugs was observed across treatment lines. The magnitude of the "new" treatment benefit on mPFS was pronounced in 1LT.
Conclusions: Forty percent of patients received therapy beyond 2lines demonstrating a great unmet need in the treatment of RRMM. While BTZ and LEN were predominant in 1st and 2nd lines, substantial fragmentation was seen in ≥3 line, highlighting the lack of defined treatment pathways for these patients. The results further indicated that treatments beyond 2LT offer shorter benefit as disease progresses; median time on treatment and mPFS decreased as treatment line increased. Also, median PFS and OS in this real-world analysis were also slightly lower than that observed in recent clinical trials of novel agents, which is consistent with other real-world studies. While there remains a need to replicate these results within a larger dataset where statistical comparisons could be made and confounding factors controlled for, the trends observed in this study suggest improved PFS and OS outcomes may be associated with newly approved treatments. In particular, the trends suggested that newer treatments may have a greater mPFS benefit vs. older treatments if used in earlier lines of treatment.
Funding: GSK (HO-17-17767)
Willson:GlaxoSmithKline: Employment, Equity Ownership. Bruno:GlaxoSmithKline: Employment, Equity Ownership. Opalinska:GlaxoSmithKline: Employment, Equity Ownership. Nelson:GlaxoSmithKline: Equity Ownership. Stafkey-Mailey:Xcenda: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal